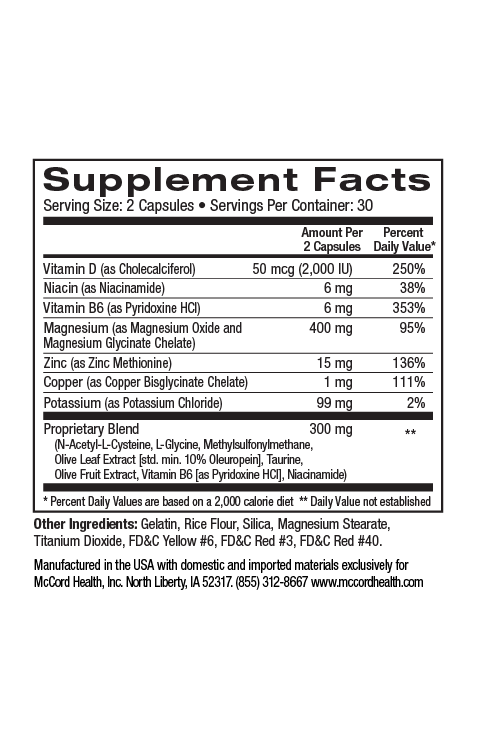
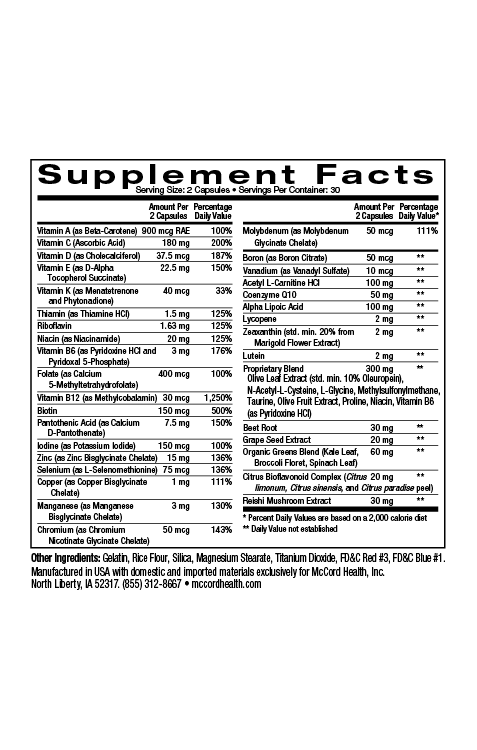
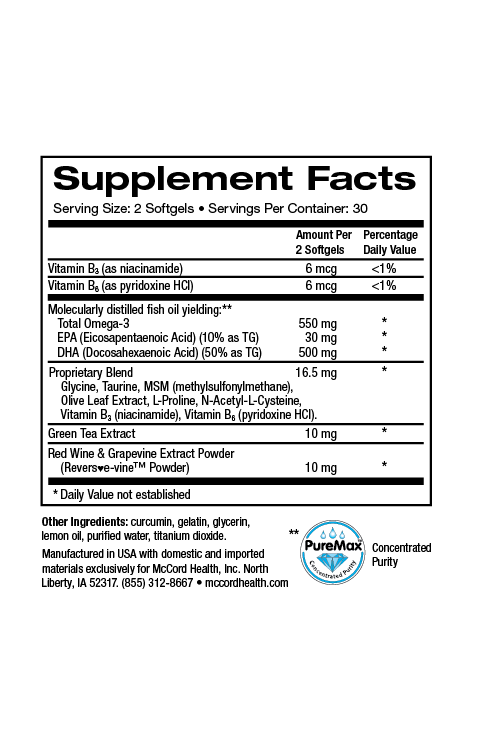
Hyaluronic Acid (HA), also known as hyaluronan, HA is one of the most ubiquitous linear polysaccharides found in nature. It is found in almost every part of the body including skin. It’s an important component of the extracellular matrix (ECM), a complex interlocking meshwork found outside of skin cells (and other cells) that includes fibrous proteins such as collagens, elastin and fibronectin. Connective tissues including skin and cartilage are especially rich in ECM. As an integral part of the ECM, HA provides a structural role, as well as many other crucial roles. Amazingly, approximately half of the body’s total HA is located in skin. Fibroblasts produce most of the HA in humans. In addition, epidermal keratinocytes make some HA.
HA consists of numerous repeating disaccharide units of glucuronic acid and N-acetylglucosamine giving it a molecular weight of up to several million daltons. Interestingly, high molecular weight HA is deposited in skin during homeostasis where it promotes skin stability. One way that HA promotes stability is through its interactions with other ECM components, which facilitates proper skin tissue organization. HA like other ECM components is also critical in the regulation of all stages of wound repair including cellular migration, inflammation and remodeling.
Viniferamine® skin and wound care products include small molecule ingredients that protect HA. Many ingredients decrease excess inflammation and increase wound healing including the powerful anti-inflammatory polyphenols oleuropein, resveratrol, and epigallocatechin-3-gallate (EGCG) from olives, grapes, and green tea, respectively, as well as the important anti-inflammatory small molecules, melatonin, and L-glutathione. In addition, dipotassium glycyrrhizate from licorice, avenanthramides in oats, aloe vera and shea butter possess anti-inflammatory activities. Oleuropein, L-carnosine, L-glutathione, asiaticoside and aloe vera improve wound healing along with titrated extract of Centella asiatica (TECA) and aloe vera that stimulate collagen synthesis.
Its large size and charge play an important role in HA modulation of ionic and molecular traffic through the ECM that contributes to cellular signaling. In fact, HA excludes tissue degrading enzymes from other structural components of the ECM. In addition, HA scavenges free radicals and serves as an important antioxidant in skin, which may be particularly important for protection against solar radiation damage. Furthermore, HA is anti-inflammatory. In comparison, aged skin, due to exposure to UV (photoaged), has diminished levels of HA leading to skin atrophy. UV radiation also produces HA fragments (low molecular weight HA), which in contrast are inflammatory.
In younger or protected skin (due to its negative charge and high molecular weight), HA has a large capacity for binding water and electrolytes, which greatly enhances skin hydration and health. However, as mentioned, HA is depleted even during normal aging, which results in less skin volume and skin wrinkling, due to decreased skin hydration as well as a decreased ability of aging skin to produce collagen. Aging can also lead to smaller HA molecules as a result of increased levels of the degradative enzyme, hyaluronidase and the production of free radicals known as reactive oxygen species (ROS) that increase with aging, leading to oxidative stress. In addition, some drugs including glucocorticoids like hydrocortisone can decrease levels of HA in any skin with prolonged use leading to skin thinning and atrophy.
Viniferamine® skin and wound care products including Renewal Moisturizer and Silicone Barrier contain dipotassium glycyrrhizate and aloe vera, which have been found to protect HA from degradation. Oxidative stress results from the inability of cells to eliminate ROS using the natural defense system that includes defense enzymes such as superoxide dismutase (SOD). Viniferamine® skin and wound products contain potent antioxidants such as oleuropein, resveratrol, and EGCG, as well as melatonin, and L-glutathione. In fact, in a model where manganese (Mn)SOD was deactivated, oleuropein induced MnSOD activity; EGCG has been found to induce MnSOD expression and resveratrol has been shown to upregulate MnSOD activity.
It’s good to know that Viniferamine® skin and wound care products contain ingredients that protect HA from degradation to help increase skin health, hydration and stability. In addition, Viniferamine® products contain many beneficial ingredients that decrease inflammation and oxidative stress and increase wound healing.
About the author: Nancy Ray, PhD is the Science Officer at McCord Research. Dr. Ray received her PhD in Biochemistry and Biophysics and was a postdoctoral fellow at NIH, Harvard University and Dana-Farber Cancer Institute, and the University of Iowa. She also earned bachelor of science degrees in Chemistry and Microbiology.
References
- Food and Chem Toxicol 2017; 101: 128-138.
- Wounds 2016; 28: 78-88.
- Exp Dermatol 2014; 23: 295-303.
- Dermato-Endocrinol 2012; 4: 253-258.
- Wound Rep Reg 2014; 22: 579-593.
- Int J Mol Sci 2014; 15: 18508-18524.
- Diab Vasc Dis Res 2014; 11: 92-102.
- Oxid Med Cell Longev 2012; ID 560682:1-8.
- J Pineal Res 2013; 55: 325-356.
- Int J Gen Med 2011; 4: 105-113.
- Evid Based Complement Altern Med 2012; ID 650514:1-9.
- Cell J 2014; 16: 25-30.
- ISRN Endicronol 2014; ID 816307: 1-8.
- J Am Acad Dermatol 2005; 52: 1049-1059.
- J Pineal Res 2008; 44: 387-396.
- Surgery 1986; 100: 815-821.
- Ann Plast Surg 2007; 58: 449-455.
- Phytother Res 1999; 13: 50-54.
- J Ethnopharmacol 1998; 59: 179-186.
- Connect Tissue Res 1990; 24: 107-120.
- BMC Compl Altern Med 2012; 12: 103-120.
- Altern Med Rev 2003; 8: 359-377.
- Curr Med Chem 2009; 16: 2261-2288.
- Evidence-Based Compl Alt Med 2012; 2012: ID 650514.
- Int J Pharm 2002; 241: 319-327.
- Int J Biochem Cell Biol 2008; 40: 1101-1110.
- Ann Plast Surg 2007; 58: 449-455.
- PLOS One 2015; 10: e0115341: 1-18.
- Exp Dermatol 2008; 17: 713-730.
- J Biol Regul Homeost Agents 2014; 28: 105-116.
- Arch Biochem Biophys 2008; 171-177.
- Phytochem 2014; 98: 164-173.
Disclaimer: These statements have not been reviewed by the FDA. The decision to use these products should be discussed with a trusted healthcare provider. The authors and the publisher of this work have made every effort to use sources believed to be reliable to provide information that is accurate and compatible with the standards generally accepted at the time of publication. The authors and the publisher shall not be liable for any special, consequential, or exemplary damages resulting, in whole or in part, from the readers’ use of, or reliance on, the information contained in this article. The publisher has no responsibility for the persistence or accuracy of URLs for external or third party Internet websites referred to in this publication and does not guarantee that any content on such websites is, or will remain, accurate or appropriate.