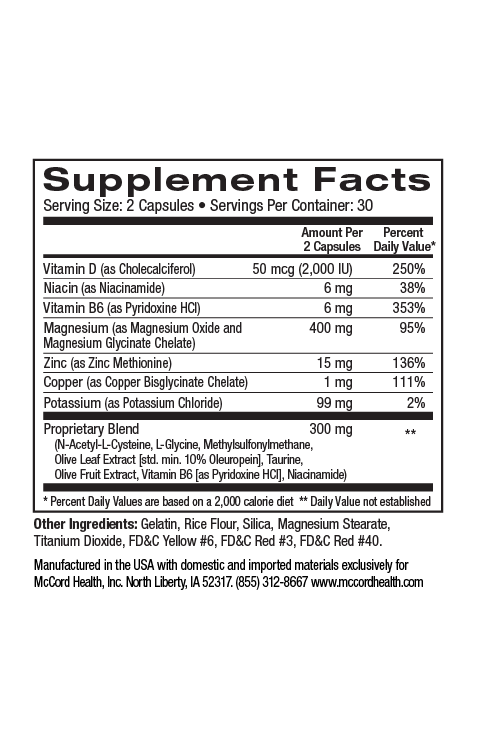
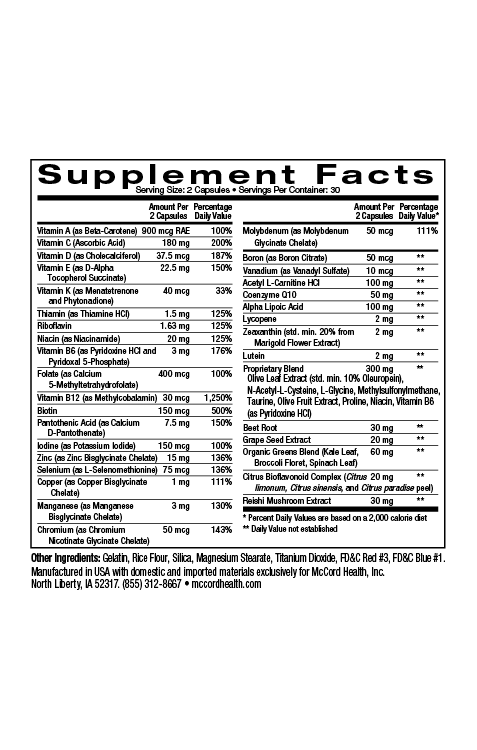
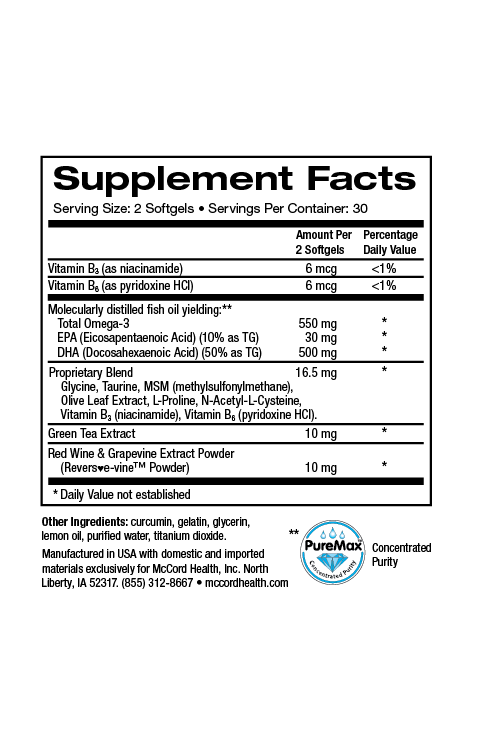
Approximately 1-2% of people develop chronic wounds in their lifetime. It’s estimated that in the United States, around 6.5 million patients experience chronic wounds resulting in close to $25 billion dollars spent annually for their treatment. Chronic wounds are wounds that have failed to proceed through the normal wound repair (healing) process for 3 months or more. Typical chronic wounds include diabetic foot ulcers, venous leg ulcers, pressure ulcers and ulcers resulting from peripheral vascular disease. Chronic wounds are extremely complex and have a multitude of potential influencing factors including changes in pH, defective extracellular matrix (ECM) synthesis, chronic inflammation and increased protease production, as well as biofilm formation. In fact, in a recent study, 60% of the chronic wounds examined contained biofilms.
Biofilms are three-dimensional aggregates of densely packed microbes encased in an extracellular polymeric substance (EPS) that attach to one another, frequently adhering tightly to surfaces including skin. Many chronic infections and diseases are associated with biofilms including cystic fibrosis, periodontitis, endocarditis, and chronic wounds. In fact, more than 65% of microbial infections are associated with biofilms. In addition, it has been shown that most biofilm-forming bacteria and yeast (including Candida albicans) cause chronic infections (including wound infections) characterized by persistent inflammation and tissue damage. Evidence suggests that effective targeting of biofilm bacteria in chronic wounds is an important component of transforming non-healing wounds into healing wounds.
Biofilm-forming microbes display characteristics that are quite different from their free-living (planktonic) counterparts including the ability to produce signaling molecules that allow them to act as a multicellular entity. The ability of bacteria to communicate via quorum sensing has been proposed to be involved in the transformation of bacteria from planktonic to biofilm forms. The growth phase of a bacterial biofilm typically involves the synergistic ability of different bacterial species to work together and encase themselves in an EPS using quorum sensing. Along with other factors, low oxygen and metabolic activities inside the biofilm architecture correlate with the ability of biofilms to resist antibiotics (and antifungals).
Viniferamine® skin and wound care products contain ingredients with various antimicrobial activities including activities against biofilms. Oleuropein from olives has activity against several wound microorganisms. Resveratrol from grapes has activity against biofilms formed by Staphylococcus aureus, Pseudomona aeruginosa and Vibrio cholera (rarely but sometimes found in wound infections) and EGCG from green tea that has activity against E. coli and C. albicans biofilms, as well as activity that disrupts the quorum sensing pathogenicity of P. aeruginosa. Furthermore, chlorhexidine digluconate found in Viniferamine® Silicone Barrier has activity against biofilms formed by Staphylococcus epidermidis and P. aeruginosa, as well as C. albicans. In addition, benzalkonium chloride found in Viniferamine® Antiseptic Cleanser has activity against E. coli, Klebsiella pneumonia, and S. epidermidis biofilms.
Normal wounds typically heal following overlapping phases including hemostasis, inflammation, proliferation and remodeling. Chronic wounds get stuck in the inflammatory phase inhibiting re-epithelialization. One of the reasons this may occur relates to the fact that during the inflammatory response to biofilm infection, immune cells are unable to reach the microbes to kill them since the biofilm microbes are protected by their EPS. Instead, white blood cells attach to the outside of biofilms and die, releasing enzymes and DNA that increase and prolong the inflammatory response.
Viniferamine® skin and wound care products include potent anti-inflammatory small molecule ingredients including the beneficial polyphenols oleuropein, resveratrol, and epigallocatechin-3-gallate (EGCG) from olives, grapes, and green tea, respectively, as well as the important small molecules, melatonin, and L-glutathione. In addition, dipotassium glycyrrhizinate from licorice, aventhramides in oats, aloe vera and shea butter have also been shown to possess anti-inflammatory activities.
Skin provides a fairly inhospitable environment for most microbes due to its ability to remain cool, dry and slightly acidic. The normal pH of skin ranges between 4.0 and 5.5. Maintaining this pH range in skin is critical for the maintenance of a healthy skin microbiome. The normal skin microbiome is composed of a rich and complex flora of interacting microbes that live in harmony with skin protecting it from potentially dangerous pathogens. When a wound occurs, the natural acidity of the skin is disrupted and the more neutral pH (7.4) of the underlying tissue is exposed, then with normal wound healing, there is a decrease in pH in the wound due to various factors including hypoxia and greater lactic acid production.
When wound healing is delayed (as with a chronic wound), the pH oscillates and becomes increasingly alkaline. The synthesis of ECM molecules is impaired and the healing process is arrested. Chronic wound environments have exhibited pH values in the range of 7.15 to 8.9. Bacterial colonization may contribute to the shift toward an alkaline pH in chronic wounds. One study showed that increasing pH correlated with an increase in biofilm production by all of the microorganisms they examined. In fact, significantly higher biofilm production was found with a pH of 7.5 and 8.5 compared with a pH of 5.5.
Viniferamine® skin and wound products, including Antiseptic Cleanser and Silicone Barrier are pH balanced to preserve the skin’s normal chemistry and flora and protect compromised skin from the risk of infection. Antiseptic Cleanser includes a gentle, broad-spectrum antimicrobial to reduce overpopulation of microorganisms and reduce the risk of skin infections. Silicone Barrier is an advanced silicone cream that provides a “second skin” to protect compromised epidermis. Silicone Barrier contains a sophisticated silicone complex and ingredients proven to protect compromised skin from the risk of infection.
It’s good to know that Viniferamine® skin and wound care products include ingredients that help decrease inflammation and the risk of developing infections, biofilms and chronic wounds. In addition, Viniferamine® Antiseptic Cleanser and Silicone Barrier help maintain the skin’s normal slightly acidic pH to further help reduce the risk of infections. Moreover, Viniferamine® skin and wound care products contain skin nutrients including antioxidants, amino acids, and vitamins to strengthen skin and help prevent wounding.
About the author: Nancy Ray, PhD is the Science Officer at McCord Research. Dr. Ray received her PhD in Biochemistry and Biophysics and was a postdoctoral fellow at NIH, Harvard University and Dana-Farber Cancer Institute, and the University of Iowa. She also earned bachelor of science degrees in Chemistry and Microbiology.
References
- Wound Rep Regen 2009; 17: 763-771.
- Adv Wound Care 2013; 2: 389-399.
- Wound Rep Regen 2013; 21: 352-362.
- Ann Plast Surg 2015; 00: 1-5.
- Biofoul 2015: 31: 1-11.
- Future Med Chem 2015; 7: 647-671.
- Phytomedicine 2014; 21: 286-289.
- Z Naturforsch 2003; 58c: 879-884.
- J Biochem Pharmacol Res 2014; 2: 167-174.
- Can J Microbiol 2009; 55: 1033-1039.
- Sci Rep 2015; 5:16158. doi:10.1038/srep16158.
- J Antimicrob Chemother 2008; 62: 1031-1036.
- Skin Pharmacol Physiol 2010; 23Suppl: 28-34.
- Antimicrob Agents Chemother 2002; Nov: 3522-3531.
- J Applied Microbiol 2008; 105: 1310-1317.
- Lett Applied Microbiol 2007: 45: 652-656.
- Int J Mol Sci 2014; 15: 18508-18524.
- Diab Vasc Dis Res 2014; 11: 92-102.
- Oxid Med Cell Longev 2012; ID 560682:1-8.
- J Pineal Res 2013; 55: 325-356.
- Int J Gen Med 2011; 4: 105-113.
- Evid Based Complement Altern Med 2012; ID 650514:1-9.
- Br J Gen Pract 1999; 49: 823-828.
- Arch Derm Res 2008; 300: 569-574.
- J Oleo Sci 2010; 59: 273-280.
- ISRN Endicronol 2014; 2104: 816307.
- J Am Acad Dermatol 2005; 52: 1049-1059.
- Adv Wound Care 2015; 4: 431-439.
Disclaimer: These statements have not been reviewed by the FDA. The decision to use these products should be discussed with a trusted healthcare provider. The authors and the publisher of this work have made every effort to use sources believed to be reliable to provide information that is accurate and compatible with the standards generally accepted at the time of publication. The authors and the publisher shall not be liable for any special, consequential, or exemplary damages resulting, in whole or in part, from the readers’ use of, or reliance on, the information contained in this article. The publisher has no responsibility for the persistence or accuracy of URLs for external or third party Internet websites referred to in this publication and does not guarantee that any content on such websites is, or will remain, accurate or appropriate.