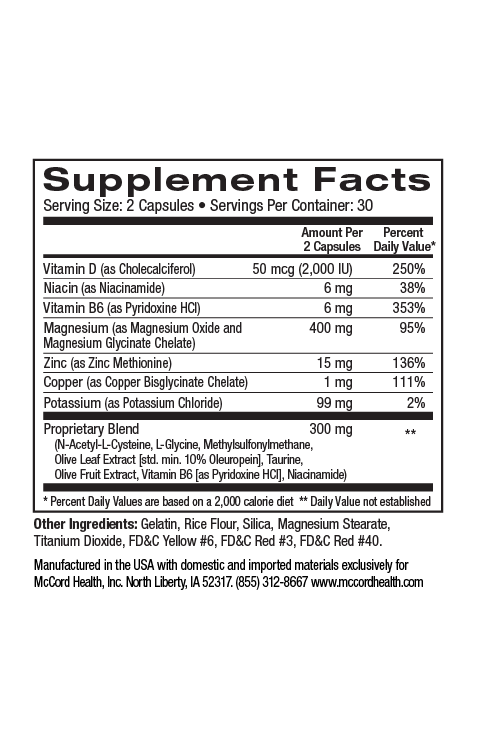
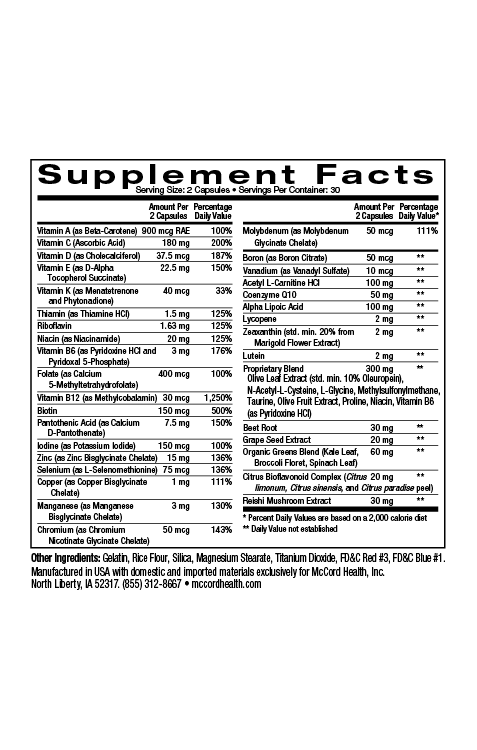
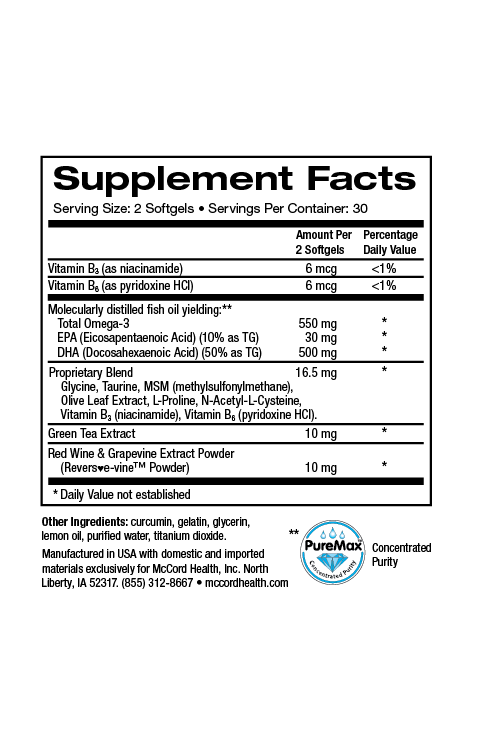
Parabens were first used in the mid1920s for their antimicrobial properties. By the early 2000s, parabens were found in over 90% of cosmetic products. Parabens are a group of alkyl esters of p-hydroxybenzoic acid (PHBA) including methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben, isopropylparaben and benzylparaben. Paraben compounds are either used alone or as a mixture in products to increase their efficacy. In fact, currently, parabens are widely used as preservatives in many products including cosmetics, skincare, toothpaste, underarm deoderants, pharmaceuticals and food packaging products, in part due to their low cost, as well as lack of color and odor.
Viniferamine® skin and wound care products are paraben-free. Instead, they include a natural, organic preservative system based on certified organic citrus oils. This preservative system that meets the United States Pharmacopeia (USP) standards is both effective and safe. In addition, the preservative system has a pleasant fragrance that contributes to the elegant and enjoyable overall fragrance of the Viniferamine® products.
Some studies have indicated that parabens have a prooxidant activity in skin, which could lead to decreased levels of the important antioxidant, glutathione and result in oxidative stress. In fact, methylparaben was found to have a detrimental impact on human skin keratinocytes when combined with UVB light exposure, and studies have shown that UVB radiation-induced reactive oxygen species (ROS) production was significantly enhanced by methyparaben.
Oxidative stress that typically leads to inflammation results from the inability of cells to eliminate free radicals known as ROS using the natural defense system that includes antioxidants and defense enzymes such as manganese superoxide dismutase (MnSOD). Various ingredients in Viniferamine® skin and wound products counteract oxidative stress including oleuropein, resveratrol, and EGCG, as well as melatonin, and L-glutathione. In fact, in a model where MnSOD was deactivated, oleuropein induced MnSOD activity; EGCG has been found to induce MnSOD expression, and resveratrol has been shown to upregulate MnSOD activity.
In addition, many of the small molecule ingredients found in Viniferamine® skin and wound care products have potent anti-inflammatory activities to decrease skin inflammation including the polyphenols oleuropein, resveratrol, and epigallocatechin-3-gallate (EGCG) from olives, grapes, and green tea, respectively, as well as the important small molecules, melatonin, and L-glutathione. Moreover, dipotassium glycyrrhizate from licorice, avenanthramides in oats, aloe vera and shea butter possess anti-inflammatory activities.
The application of skin products containing parabens has resulted in allergic contact dermatitis, especially in patients with eczema. In fact, contact sensitization to parabens occurs in approximately 0.5% to 3.5% of patients who attend contact sensitization clinics. Viniferamine® skin and wound products include non-sensitizing ingredients that can be applied to skin daily without irritation even in patients prone to skin allergies. In fact, several of the ingredients in Viniferamine® products strengthen the skin barrier to help patients with eczema decrease the occurrence of skin reactions caused by exposure to environmental irritants.
Parabens have endocrine disrupting effects that include alteration of natural hormone action as well as changes in hormone synthesis. In fact, parabens can be absorbed through the skin intact and are known to have estrogenic activity. Accumulating evidence suggests that dermal absorption of estrogenic chemicals applied in personal care products to the underarm and breast region may be involved in the development of breast cancer. Parabens also have estrogen-independent mechanisms of action that alter cell homeostasis including promoting the growth of breast cancer cells lacking estrogen receptors. Moreover, parabens have the ability to alter the processes of spermatogenesis in experimental models.
As mentioned, parabens can be absorbed by human skin and the measurement of intact parabens in human tissues demonstrates the ability of these compounds to escape metabolism by skin esterases. Personal care products applied on a frequent basis and left on the skin continuously may lead to absorption and accumulation of parabens in underlying tissues. Amazingly, parabens have been measured in the blood in as little as one hour after human dermal exposure.
It may be difficult to keep track of which products contain parabens and at what levels, but with Viniferamine® skin and wound care products one can rest assured that application of Viniferamine® products to the skin will not result in increased levels of parabens in the body that can accumulate and result in local and systemic effects.
It’s good to know that Viniferamine® skin and wound care products including Renewal Moisturizer and Silicone Barrier contain a certified organic citrus oil based preservative system instead of paraben, eliminating the risk of skin exposure to this potentially harmful chemical. Viniferamine® products are paraben-free so that various skin issues or wounds can be addressed effectively and as frequently as necessary without the possible side effects caused by unsafe preservatives.
About the author: Nancy Ray, PhD is the Science Officer at McCord Research. Dr. Ray received her PhD in Biochemistry and Biophysics and was a postdoctoral fellow at NIH, Harvard University and Dana-Farber Cancer Institute, and the University of Iowa. She also earned bachelor of science degrees in Chemistry and Microbiology.
References
- Dermatitis 2015; 26: 254-259.
- Crit Rev Toxicol 2005; 35: 435-458.
- Environ Res 2016; 151: 30-37.
- Dermatitis 2014; 25: 215-231.
- Environ Res 2016; 151: 251-264.
- J Appl Toxicol 2014; 34: 925-938.
- Environ Int 2014; 67: 27-42.
- Int J Mol Sci 2014; 15: 18508-18524.
- Oxid Med Cell Longev 2012; ID 560682:1-8.
- Ann Plast Surg 2007; 58: 449-455.
- PLOS One 2015; 10: e0115341: 1-18.
- Exp Dermatol 2008; 17: 713-730.
- J Biol Regul Homeost Agents 2014; 28: 105-116.
- Arch Biochem Biophys 2008; 171-177.
- Phytochem 2014; 98: 164-173.
- Diab Vasc Dis Res 2014; 11: 92-102.
- J Pineal Res 2013; 55: 325-356.
- Int J Gen Med 2011; 4: 105-113.
- Evid Based Complement Altern Med 2012; ID 650514:1-9.
- Cell J 2014; 16: 25-30.
- ISRN Endicronol 2014; ID 816307: 1-8.
- J Am Acad Dermatol 2005; 52: 1049-1059.
Disclaimer: These statements have not been reviewed by the FDA. The decision to use these products should be discussed with a trusted healthcare provider. The authors and the publisher of this work have made every effort to use sources believed to be reliable to provide information that is accurate and compatible with the standards generally accepted at the time of publication. The authors and the publisher shall not be liable for any special, consequential, or exemplary damages resulting, in whole or in part, from the readers’ use of, or reliance on, the information contained in this article. The publisher has no responsibility for the persistence or accuracy of URLs for external or third party Internet websites referred to in this publication and does not guarantee that any content on such websites is, or will remain, accurate or appropriate.